Blog de cursos y estudiantes de Químicas del Departamento de Ciencias Quimico-Biológicas en la Universidad de las Américas Puebla.
Sunday, March 25, 2012
Exploring the coordination chemistry of MOF-graphite oxide composites and their applications as adsorbents.

Towards a New Family of Photoluminescent Organozinc 8-Hydroxyquinolinates with a High Propensity to Form Noncovalent Porous Materials.
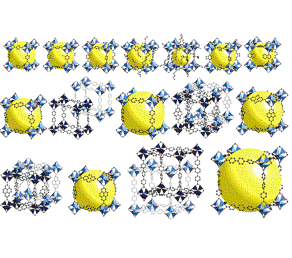
 We report on investigations of reactions of tBu(2) Zn with 8-hydroxyquinoline (q-H) and the influence of water on the composition and structure of the final product. A new synthetic approach to photoluminescent zinc complexes with quinolinate ligands was developed that allowed the isolation of a series of structurally diverse and novel alkylzinc 8-hydroxyquinolate complexes: the trinuclear alkylzinc aggregate [tBuZn(q)](3) (1(3) ), the pentanuclear oxo cluster [(tBu)(3) Zn(5) (μ(4) -O)(q)(5) ] (2), and the tetranuclear hydroxo cluster [Zn(q)(2) ](2) [tBuZn(OH)](2) (3). All compounds were characterized in solution by (1) H NMR, IR, UV/Vis, and photoluminescence (PL) spectroscopy, and in the solid state by X-ray diffraction, TGA, and PL studies. Density functional theory calculations were also carried out for these new Zn(II) complexes to rationalize their luminescence behavior. A detailed analysis of the supramolecular structures of 2 and 3 shows that the unique shape of the corresponding single molecules leads to the formation of extended 3D networks with 1D open channels. Varying the stoichiometry, shape, and supramolecular structure of the resulting complexes leads to changes in their spectroscopic properties. The close-packed crystal structure of 1(3) shows a redshifted emission maximum in comparison to the porous crystal structure of 2 and the THF-solvated structure of 3.
We report on investigations of reactions of tBu(2) Zn with 8-hydroxyquinoline (q-H) and the influence of water on the composition and structure of the final product. A new synthetic approach to photoluminescent zinc complexes with quinolinate ligands was developed that allowed the isolation of a series of structurally diverse and novel alkylzinc 8-hydroxyquinolate complexes: the trinuclear alkylzinc aggregate [tBuZn(q)](3) (1(3) ), the pentanuclear oxo cluster [(tBu)(3) Zn(5) (μ(4) -O)(q)(5) ] (2), and the tetranuclear hydroxo cluster [Zn(q)(2) ](2) [tBuZn(OH)](2) (3). All compounds were characterized in solution by (1) H NMR, IR, UV/Vis, and photoluminescence (PL) spectroscopy, and in the solid state by X-ray diffraction, TGA, and PL studies. Density functional theory calculations were also carried out for these new Zn(II) complexes to rationalize their luminescence behavior. A detailed analysis of the supramolecular structures of 2 and 3 shows that the unique shape of the corresponding single molecules leads to the formation of extended 3D networks with 1D open channels. Varying the stoichiometry, shape, and supramolecular structure of the resulting complexes leads to changes in their spectroscopic properties. The close-packed crystal structure of 1(3) shows a redshifted emission maximum in comparison to the porous crystal structure of 2 and the THF-solvated structure of 3.Kamil Sokołowski, Iwona Justyniak, Witold Sliwiński, Katarzyna Sołtys, Adam Tulewicz, Arkadiusz Kornowicz, Robert Moszyński, Janusz Lipkowski, Janusz Lewiński.
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw (Poland), Fax: (+48) 22-3433333.
Journal Article: Chemistry (impact factor: 5.38). 03/2012; DOI: 10.1002/chem.201104028
Monday, March 12, 2012
Hydrogen Bonding Patterns and Supramolecular Structure of 4,4′-Bipyrazolium Salts

12/marzo/2012
Maximiliano De La Higuera Macías
The crystal structures of 18 inorganic salts of 4,4′-bipyrazolium [H2bpz]2+ and 3,3′,5,5′-tetramethyl-4,4′-bipyrazolium [H2Me4bpz]2+ (bpz = 4,4′-bipyrazole; Me4bpz = 3,3′,5,5′-tetramethyl-4,4′-bipyrazole) involving Cl−, I−, I3−, PdCl42−, Cu2Cl62−, Re2Cl82−, SiF62−, TaF6−, Zr2F124−, (BeF3−)n, IO3−, ClO4−, S2O62−, HSO4−, and H2PO4− ions were determined by X-ray diffraction. Primary supramolecular organization of the bipyrazolium salts originates in strong hydrogen bonding between multiple NH cationic donors and O, F, Cl, I anionic acceptors following three main modes, which support linear joints of the cationic moieties: {(Hpz+)2(A−)2}, {(Hpz+)2(AX2−)2}- two pyrazolium moieties joined by a one-atom and three-atom bridging fragment respectively, and {(Hpz+)(AX2−)} - a single pyrazolium moiety “capped” by a three-atom anionic fragment. These modes provide suitable and characteristic supramolecular synthons for the rational design of hydrogen bonded pyrazolium frameworks. The control over dimensionality of the structure is feasible through proper choice of the anion, its charge, and configuration of the acceptor atoms. A relatively high number of hydrogen bond acceptor atoms of the anions (TaF6−, Zr2F124−, ClO4−) results in bifurcation of NH···X bonding. Weaker CH···X hydrogen bonding and slipped π/π interactions are relevant for the secondary supramolecular organization.
Ishtvan Boldog (et al). Hydrogen Bonding Patterns and Supramolecular Structure of 4,4′-Bipyrazolium Salts Inorganic Chemistry Department, Kiev University, Volodimirska Street 64, Kiev 01033, Ukraine, LCC Toulouse, 205, Route de Narbonne, 31077 Toulouse Cedex 4, France, Institute of Organic Chemistry, Murmanskaya Str. 4, 253660, Ukraine, and Institut für Anorganische Chemie, Universität Leipzig, Linnéstraβe 3, D-04103 Leipzig, Germany
Cryst. Growth Des., 2009, 9 (6), pp 2895–2905
DOI: 10.1021/cg9002109
http://pubs.acs.org/doi/pdf/10.1021/cg9002109
Lone Pair Effect in Thallium(I) Macrocyclic Compounds
Maximiliano De La Higuera Macías
 The role of the inert (lone) pair of electrons in thallium(I) salts is studied by comparison of the compounds [Tl@18-crown-6]+X- (X = TlI4, ClO4) and [K@18-crown-6]+ClO4-. In contrast to common introductory chemistry textbook opinions, the paradigm that s−p hybridization is a prerequisite for an inert electron pair to become stereochemically active in compounds of the heavier main group elements has to be revised. Instead, an inert pair of electrons is expected to become stereochemically involved whenever it is forced to participate in antibonding orbital interactions with its surroundings, and there is the possibility for a structural distortion that minimizes these repulsive forces. The structural distortion will occur to such an extent that repulsive orbital interactions and attractive electrostatic interactions counterbalance. Our results also provide an explanatory background for many of the rules of thumb that are found in the literature about why and when an inert electron pair is expected to become stereochemically active in a certain compound.
The role of the inert (lone) pair of electrons in thallium(I) salts is studied by comparison of the compounds [Tl@18-crown-6]+X- (X = TlI4, ClO4) and [K@18-crown-6]+ClO4-. In contrast to common introductory chemistry textbook opinions, the paradigm that s−p hybridization is a prerequisite for an inert electron pair to become stereochemically active in compounds of the heavier main group elements has to be revised. Instead, an inert pair of electrons is expected to become stereochemically involved whenever it is forced to participate in antibonding orbital interactions with its surroundings, and there is the possibility for a structural distortion that minimizes these repulsive forces. The structural distortion will occur to such an extent that repulsive orbital interactions and attractive electrostatic interactions counterbalance. Our results also provide an explanatory background for many of the rules of thumb that are found in the literature about why and when an inert electron pair is expected to become stereochemically active in a certain compound. Anja-Verena Mudring* and Franziska Rieger. Institut für Anorganische Chemie, Universität zu Köln, Greinstrasse 6, D-50939 Köln, Germany Lone Pair Effect in Thallium(I) Macrocyclic Compounds. Inorg. Chem., 2005, 44 (18), pp 6240–6243 August 5, 2005
DOI: 10.1021/ic050547k
http://pubs.acs.org/doi/pdf/10.1021/ic050547k
Co4(OH)2(C10H16O4)3 Metal–Organic Framework: Slow Magnetic Relaxation in the Ordered Phase of Magnetic Chains
 12/marzo/2012
12/marzo/2012Maximiliano De La Higuera Macías
Reported here are the synthesis and structural and topological analysis as well as a magnetic investigation of the new Co4(OH)2(C10H16O4)3 metal−organic framework. The structural analysis reveals a one-dimensional inorganic subnetwork based on complex chains of cobalt(II) ions in two different oxygen environments. Long alkane dioic acid molecules bridge these inorganic chains together to afford large distances and poor magnetic media between dense spin chains. The thermal dependence of the χT product provides evidence for uncompensated antiferromagnetic interactions within the cobaltous chains.
In zero-field, dynamic magnetic susceptibility measurements show slow magnetic relaxation below 5.4 K while both neutron diffraction and heat capacity measurements give evidence of long-range order (LRO) below this temperature. The slow dynamics may originate from the motion of broad domain walls and is characterized by an Arrhenius law with a single energy barrier Δτ/kB = 67(1) K for the [10−5000 Hz] frequency range.
Moreover, in nonzero dc fields the ac susceptibility signal splits into a low-temperature frequency-dependent peak and a high-temperature frequency-independent peak which strongly shifts to higher temperature upon increasing the bias dc field. Heat capacity measurements have been carried out for various applied field values, and the recorded CP(T) data are used for the calculation of the thermal variations of both the adiabatic temperature change ΔTad and magnetic entropy change ΔSm. The deduced data show a modest magnetocaloric effect at low temperature. Its maximum moves up to higher temperature upon increasing the field variation, in relation with the fieldsensibility of the intrachain magnetic correlation length.
Romain Sibille, Thomas Mazet, Bernard Malaman, Thomas Gaudisson, and Michel François
Institut Jean Lamour, UMR 7198—Nancy Université, BP 70239, 54506 Vandoeuvre-lès-Nancy Cedex, France. Co4 (OH) 2(C10H16O4)3 Metal–Organic Frameworks: Slow Magnetic Relaxation in the Ordered Phase of Magnetic Chains Inorg. Chem., 2012, 51 (5), pp 2885–2892
DOI: 10.1021/ic2020995. February 17, 2012
http://pubs.acs.org/doi/pdf/10.1021/ic2020995
Hybrid Structure of Zinc Oxide Nanorods and Three Dimensional Graphene Foam for Supercapacitor and Electrochemical Sensor
 12/marzo/2011
12/marzo/2011 Maximiliano De La Higuera Macías
A hybrid structure of zinc oxide (ZnO) on three dimensional (3D) graphene foam has been synthesized by chemical vapor deposition (CVD) growth of graphene followed by a facial in-situ precipitation of ZnO nanorods under hydrothermal conditions. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) are used to characterize the morphology and structure of graphene/ZnO hybrids. The results show that the ZnO nanorods have high crystallinity and cluster uniformly on graphene skeleton to form flower-like nanostructures. Serving as a free-standing electrode, the electrochemical and biosensing performance of graphene/ZnO hybrid are studied by cyclic voltammetry, electrochemical impedance spectroscopy, galvanostatic charge/discharge and amperometric measurements.
Peng Chen. Hybrid Structure of Zinc Oxide Nanorods and Three Dimensional Graphene Foam for Supercapacitor and Electrochemical Sensor Applications. RSC Advances. Royal Society of Chemistry. Mar 2, 2012. http://pubs.rsc.org/en/content/articlepdf/2012/ra/c2ra01295b
NEODIMIO ¡no te lo pierdas!

-
De entre todos los compuestos químicos que existen, quizá sean los llamados compuestos de coordinación los que mayores dificultades ofrecen ...
-
Básicamente el video habla por sí sólo. Se tiene una cerveza fría líquida recién sacada del refrigerador o congelador, se le da un golpe y e...
