Blog de cursos y estudiantes de Químicas del Departamento de Ciencias Quimico-Biológicas en la Universidad de las Américas Puebla.
Friday, April 27, 2012
Complejos de oro
átomos pesados efecto de oro (Z ¼ 79) dota Orgánicos (I) especies con
estado triplete-photoproperties entre sistemas de mejora de cruce entre
singlete y triplete estados excitados. No obstante, los fotofísica
de oro (I) arilos, es decir, de las especies aromáticas nominalmente fluorescentes
perturbado por la terminal R-bonos en oro-se hace muy poco emergente.
Fuente:http://web.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=4a1db978-ac27-45e4-b6e7-3dd8df37691e%40sessionmgr112&vid=14&hid=10
Thursday, April 26, 2012
La profundidad y la amplitud de la conexión de boro
Dos grupos se dice isolobales cuando:
- tienen la energía y la forma de la frontera orbitales moleculares similares;
- tienen simetría geométrica similar;
- el mismo número de electrones en los orbitales frontera.
Como todo esto hay una enrome esperanza de todas las aplicaciones de compuestos de carboranos y complejos y compuestos de carboranos para la Química, Medicina y Ciencia de Materiales y no solo en carboranos sino en sus análogos de Silicio-Carbono.
Fuente:http://web.ebscohost.com/ehost/pdfviewer/pdfviewer?sid=4a1db978-ac27-45e4-b6e7-3dd8df37691e%40sessionmgr112&vid=7&hid=10
Monday, April 23, 2012
Converting Graphene Oxide Monolayers into Boron Carbonitride Nanosheets by Substitutional Doping.
Nuevas herramientas para la 'nanobioingeniería'
Todos los organismos vivos estamos compuestos por células. Dentro de ellas existen una gran cantidad y variedad de macromoléculas que, organizadas en agrupaciones, realizan funciones altamente específicas tales como la translación de compuestos de un lado a otro de la célula o la replicación de otras moléculas, solo por citar algunas. El conjunto de estas agrupaciones macromoleculares compone la maquinaria celular de donde emanan todas nuestras funciones vitales: desde la nutrición o el crecimiento hasta la transmisión de la información genética de generación en generación, constituyendo la base de nuestra herencia.
Los virus, unos entes biológicos mucho más sencillos que las células, son capaces de aprovechar la maquinaria celular de estas para reproducirse. No obstante, a pesar de ser más simples, también están formados por complejas agrupaciones de macromoléculas que, por ejemplo, pueden funcionar como sofisticados, rápidos y potentes motores.
Durante los últimos 30 años, la ciencia ha dedicado un esfuerzo considerable a estudiar la estructura de estas macromoléculas. El investigador José López Carrascosa, del CNB-CSIC, ha realizado aportaciones fundamentales desarrollando nuevas herramientas para desvelar la forma tridimensional de estas importantes máquinas moleculares y su funcionamiento de forma individual.
FUENTE:
http://www.elmundo.es/elmundo/2012/01/31/nanotecnologia/1328009225.html
Llega el vendaje nanotecnológico que avisa de una infección
En condiciones normales, este singular vendaje se limita a monitorizar la herida o quemadura, a fin de detectar la proliferación indeseada de bacterias.
Si surge una infección, el vendaje libera automáticamente un agente antimicrobiano.
Si esta operación no puede detener la infección, entonces el vendaje cambia su color para alertar al paciente o al personal sanitario.
Cuando esté plenamente operativo y aprobado para su uso médico, este vendaje ayudará a combatir las infecciones en heridas y quemaduras, gracias a su actuación inmediata cuando surja una, y también a su señal de alerta temprana cuando no consiga controlarla.
El problema de la infección de heridas, especialmente con la evolución de bacterias resistentes a los antibióticos, como la Staphylococcus aureus resistente a la meticilina (MRSA, por sus siglas en inglés), es bien conocido por mucha gente, pero no así el hecho de que las infecciones de heridas son la causa específica de muerte de la mitad de todas las personas que fallecen a consecuencia de quemaduras térmicas.
La tecnología que el equipo del Dr. Toby Jenkins, director del Grupo de Investigación de Química Biofísica en la Universidad de Bath, Reino Unido, está desarrollando, va orientada sobre todo a tratar quemaduras en niños pequeños.
Nanopills Release Drugs Directly from the Inside of Cells
UAB researchers developed a new vehicle to release proteins with therapeutic effects. This is known as "bacteria-inclusion bodies," stable insoluble nanoparticles which normally are found in recombinant bacteria. Even though these inclusion bodies traditionally have been an obstacle in the industrial production of soluble enzymes and biodrugs, they were recently recognised as having large amounts of functional proteins with direct values in industrial and biomedical applications.
The research team led by Antoni Vallverde from the Institute of Biotechnology and Biomedicine (IBB) at UAB worked in collaboration with the Online Biomedical Research Centre for Bioengineering, Biomaterials, and Nanomedicine (CIBER-BBN) to verify the value of these nanoparticles as natural "nanopills" with a strong capacity to penetrate cells and carry out biological activities. The nanopill concept represents a new and promising platform for drug administration and illustrates the yet to be explored power of microbian materials in medicine.
The researchers, in a multidisciplinary study at UAB led by Dr Esther Vàzquez, packaged four proteins containing different therapeutic effects into experimental nanopills, the inclusion bodies of the bacteria Escherichia coli. They put the bacteria in contact with cell cultures of mammals under similar conditions to those found in real clinical pathologies, "sick" cells with low viability, and achieved to recover their activity.
Once the technology was licensed to Janus Developments, the tolerance of its administration in vivo were confirmed through experiments conducted by UAB researcher Ester Fernández. The results and detailed description of the "nanopill" were published this week in the journal Advanced Materials.
The multidisciplinary study included researchers from IBB, the UAB Departments of Genetics and Microbiology and of Cell Biology, Physiology and Immunology, the CIBER-BBN, the CIBER-EHD (Online Biomedical Research Centre for Hepatic and Digestive Diseases), the firm Janus Developments, the Leibniz University of Hannover and the Helmholtz Centre for Infection Research in Germany.
The use of inclusion bodies as therapeutic agents was patented by UAB and CIBER-BBN (patent code: WO2010131117A1), and licensed to the biotechnology firm Janus Developments, which currently invests in the development of the product.
source:http://www.sciencedaily.com/releases/2012/03/120316145755.htm
Making carbon nanotube nanoelectronics safe for the environment
| (Nanowerk News) The percentage of electronic waste occupying our landfills has grown at an alarming rate over the last decade, giving rise to concerns about the toxicity of components used in consumer electronics. Researchers at the University of Florida are looking for ways to minimize environmental hazards associated with a material likely to play an increasingly important role in the manufacture of these goods in the future. The results of their most recent studies are published in the March 2012 issue of Nanotoxicology ("Mitigation of the impact of single-walled carbon nanotubes on a freshwater green algae: Pseudokirchneriella subcapitata"). | |
| Carbon nanotubes are already being used in touch screens and to make smaller, more efficient transistors. And if current research to develop them for use in lithium ion batteries is successful, carbon nanotubes could become important technology for powering everything from smartphones to hybrid vehicles. But for all of the promise developers see in this emerging technology, there is also some concern. | |
| "Depending on how the nanotubes are used, they can be toxic – exhibiting properties similar to asbestos in laboratory mice," said Jean-Claude Bonzongo, associate professor of environmental engineering at UF's College of Engineering. He is involved in a research collaboration with Kirk Ziegler, a UF associate professor of chemical engineering, to minimize this important material's potential for harm. | |
| In particular, the UF team is investigating toxicity associated with aqueous solutions of carbon nanotubes that would be used in certain manufacturing processes. | |
| "At the nano-scale, electron interactions between atoms are restricted, and that creates some of the desirable traits like the high conductivity that manufacturers want to take advantage of with carbon nanotubes," Ziegler said. "But exploiting those properties is difficult because the nanotubes tend to clump together." | |
| For that reason, carbon nanotubes have to be treated in some way to keep them dispersed and available for electron interactions that make them good conductors. One way to do it is to mix them with an aqueous solution that acts as a detergent and separates the tangled bundles. | |
| "Some of the surfactants, or solutions, are toxic on their own," Bonzongo said. "And others become toxic in the presence of carbon nanotubes." | |
| He and Zeigler are focusing their investigations on solutions that become hazardous when mixed with the carbon nanotubes. Their most recent results indicate that toxicity can be reduced by controlling the ratio of liquid to particulate. | |
| A cost-effective means of unbundling nanotubes remains one of the last hurdles for manufacturers to clear before they can employ the technology in mass-produced electronics. Current processes used for laboratory prototypes, including mechanical homogenization or centrifugal sifting, would be too expensive for manufacturing consumer electronics. For that reason, liquid suspension agents may be the way forward if we are to have nano-tech products for the masses. "It's an emerging technology," Bonzongo said. "We want to get ahead of it and make sure that the progress is sustainable — in terms of the environment and human health." | |
| Source: By Donna Hesterman, University of Florida |
Producción de gas con Nanotecnología
Según informaron los científicos en San Diego el 27 de marzo, la nueva tecnología ofrece la perspectiva de una producción más económica de una forma concentrada de gas natural con muchas de las ventajas --en cuanto a costes más bajos de almacenaje y envío-- de los conocidos concentrados de zumo de fruta congelados, detergentes líquidos para la ropa y otros productos del hogar a los que se les ha extraído el agua.
Los científicos afirmaron en la 243ª National Meeting & Exposition de la American Chemical Society (ACS), la sociedad científica más grande del mundo, que este "supergas natural
Producción de gas con Nanotecnología
Según informaron los científicos en San Diego el 27 de marzo, la nueva tecnología ofrece la perspectiva de una producción más económica de una forma concentrada de gas natural con muchas de las ventajas --en cuanto a costes más bajos de almacenaje y envío-- de los conocidos concentrados de zumo de fruta congelados, detergentes líquidos para la ropa y otros productos del hogar a los que se les ha extraído el agua.
Chips de memoria transparentes
| (Nanowerk News) Want a see-through cellphone you can wrap around your wrist? Such a thing may be possible before long, according to Rice University chemist James Tour, whose lab has developed transparent, flexible memories using silicon oxide as the active component. | |
| Tour revealed this week in a talk at the national meeting and exposition of the American Chemical Society in San Diego that the new type of memory could combine with the likes of transparent electrodes developed at Rice for flexible touchscreens and transparent integrated circuits and batteries developed at other labs in recent years. | |
| Details of the Rice breakthrough will be published in an upcoming paper, Tour said. | |
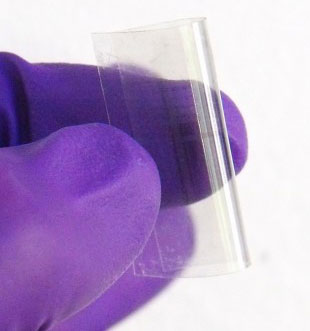 | |
| A flexible, transparent memory chip created by researchers at Rice University. (Image: Tour Lab/Rice University) | |
| "Generally, you can't see a bit of memory, because it's too small," said Tour, Rice's T.T. and W.F. Chao Chair in Chemistry as well as a professor of mechanical engineering and materials science and of computer science. "But silicon itself is not transparent. If the density of the circuits is high enough, you're going to see it." | |
| Rice's transparent memory is based upon the 2010 discovery that pushing a strong charge through standard silicon oxide, an insulator widely used in electronics, forms channels of pure silicon crystals less than 5 nanometers wide. The initial voltage appears to strip oxygen atoms from the silicon oxide; lesser charges then repeatedly break and reconnect the circuit and turn it into nonvolatile memory. A smaller signal can be used to poll the memory state without altering it. | |
| That discovery was reported on the front page of the New York Times. The Rice lab has since developed a working two-terminal memory device that can be stacked in a three-dimensional configuration and attached to a flexible substrate. | |
| Professor Tour gave a press conference on transparent memory chips during the convention. View a video recording of the conference here: http://www.ustream.tv/recorded/21406611. fuente: |
Sunday, April 22, 2012
Metal-interrupted perylene diimide: toward a new class of tunable n-type inorganic-organic hybrid semiconductors.
In organic thin-film transistors (OTFTs), organic electron-transport materials (n-type semiconductors) are well behind the advances in development of hole-transport materials (p-type semiconductors). Currently, one class of organic n-type semiconductor materials that is widely utilized is N,N'-dialkyl-3,4,9,10-perylenetetracarboxylic diimide (PTCDI-R) derivatives with high electron affinities (EAs), such as N,N'-dioctyl-3,4,9,10-perylenetetracarboxylic diimide with a reported EA as high as 4.4 eV. The PTCDI-R derivatives have been manipulated by adding substituents on the perylene moiety or at the amine position to afford more stable compounds and higher EAs. On the basis of these materials, we have developed metal-containing perylenediimide analogues, placing a salpen ligand for metal ion chelation between two n-isobutylnaphthalimides. We demonstrate here that the electronic properties of this class of materials can be systematically tuned in a divergent manner by simply changing the metal center. The synthesis, characterization, electrochemistry, and band-gap analysis are discussed herein.
Toward design of multiple-property inorganic-organic hybrid compounds based on face-sharing octahedral iodoplumbate chains.
we have illustrated the strategies developed to achieve inorganic-organic hybrid compounds with technologically important physical properties. A series of target inorganic-organic hybrid compounds have been accomplished by incorporating the functional organic components (with a large hyperpolarizability and luminophore Schiff base cation) into the highly polarizable one-dimensional (1-D) iodoplumbate chain network. The effect of substituent features in the phenyl ring of the Schiff base cation on its molecular conformation as well as the crystal packing structure of the hybrid compound will be discussed and the multiple physical properties (ferroelectricity, NLO and multiple band emission) will also be mentioned.
Saturday, April 21, 2012
Syntheses, Characterization, and Ethylene Polymerization of Titanium Complexes with Double-Duty Tridentate [ONN] Ligands
Role of the Alkali-Metal Cation Size in the Self-Assembly of Polyoxometalate-Monolayer Shells on Gold Nanoparticles
Thursday, April 19, 2012
novel sol-gel hybrid methyltrimethoxysilane-tetraethoxysilane as solid phase extraction sorbent for organophosphorus pesticides
A novel sol-gel hybrid methyltrimethoxysilane-tetraethoxysilane (MTMOS-TEOS) was produced and applied as sorbent for solid phase extraction (SPE). Five selected organophosphorus pesticides (OPPs) were employed as model compounds to evaluate the extraction performance of the synthesized sol-gel organic-inorganic hybrid MTMOS-TEOS. Analysis was performed using gas chromatography-mass spectrometry. Several important SPE parameters were optimized. Under the optimum extraction conditions, the method using the sol-gel organic-inorganic hybrid MTMOS-TEOS as SPE sorbent showed good linearity in the range of 0.001-1 μg L(-1), good repeatability (RSD 2.1-3.1%, n=5), low limits of detection at S/N=3 (0.5-0.9 pg mL(-1)) and limit of quantification (1-3 pg mL(-1), S/N=10). The performance of the MTMOS-TEOS SPE was compared to commercial C18 Supelclean SPE since C18 SPE is widely used for OPPs. The MTMOS-TEOS SPE method LOD was 500-600 × lower than the LOD of commercial C18 SPE. The LOD achieved with the sol-gel organic-inorganic hybrid MTMOS-TEOS SPE sorbent allowed the detection of these OPPs in drinking water well below the level set by European Union (EU) at 0.1 μg L(-1) of each pesticides. The developed MTMOS-TEOS SPE method was successfully applied to real sample analysis of the selected OPPs from several water samples and its application extended to the analysis of several fruits samples. Excellent recoveries and RSDs of the OPPs were obtained from the various water samples (recoveries: 97-111%, RSDs 0.4-2.8%, n=3) and fruit samples (recoveries: 96-111%), RSDs 1-4%, n=5) using the sol-gel organic-inorganic hybrid MTMOS-TEOS SPE sorbent. Recoveries and RSDs of OPPs from river water samples and fruit samples using C18 Supelclean SPE sorbent were 91-97%, RSD 0.9-2.6, n=3 and 86-96%, RSD 3-8%, n=5, respectively). The novel sol-gel hybrid MTMOS-TEOS SPE sorbent demonstrate the potential as an alternative inexpensive extraction sorbent for OPPs with higher sensitivity for the OPPs.
Fabrication of "strong" columnar Cu(2-x)Se superstructures assisted by inorganic ligands.
Monday, April 16, 2012
Facile preparation of magnetic C/TiO2/Ni composites and their photocatalytic performance for removal of a dye from water under UV light irradiation

Phosphorus recovery from sewage sludge with a hybrid process of low pressure wet oxidation and nanofiltration.

Sunday, April 15, 2012
Superconductors

By definition, a superconductor exhibits no resistance to electrical conductivity, and will oppose an external magnetic field, a phenomenon referred to as the Meissner effect (Figure 1) . Many pure transition metals (e.g., Ti, Zr, Hf, Mo, W, Ru, Os, Ir, Zn, Cd, Hg) and main group metals (e.g., Al, Ga, In, Sn, Pb) exhibir superconductivity, many only when esposed to high pressure conditions. These materials are referred to as Type I or soft superconductors.
Binary and ternary alloys and oxides of these elements, as well as pure V, Nb, Gd, and Tc are referred to as Type II or high-field superconductors. In contrast to Type I, these materials exhibit conductive characteristics varyng from normal metallic to superconductive, depending on the magnitude of the external magnetic field. It is noteworthy to point out that metals with the highest electrical conductivity (e.g., Cu, Au) do not naturally possess superconductivity. Although this behavior was first discover in 1911 for supercooled liquid mercury, it was not until 1957 that a theory was developed for this phenomenon.
In order to exhibit superconductive behavior, early Type I and II materials needed to be cooled below the critical temperature (Tc) ranging from 0.015 K (for W) to 23 K (for Nb3Ge). An intriguing goal of current research is to increase the Tc to room temperature ("high-temperature superconductors", HTS), which would trivialize resistence-free aplications such as power grid lines and widespread levitated trains. In 1986, Muller and Bednorz at IBN made and important discovery toward this goal -the firsr high-temperature superconductor, La2-xSrxCuO4 (LSCO), with a critical temperature of 35 K. A year later, the first material with a critical point above the boling of nitrogen (77K) was discovered, known as YBa2Cu3O7-d (YBCO), with a critical point of 92 K. In more recent years, the highest-temperature cuprate based superconductors have been synthesized with a general formula: MvNwCaxCuyOz (where M=Y, Bi, Tl, or Hg; N=Ba or Sr; v=1 or 2; w=2 or 4; x=0,1, or 2; y= 1,2, or 3; z= 3, 4, 6, 7, 9, 10, or 15). To date, the highest temperature superconductived materials are thallium (e.g., TlBa2Ca2Cu3O9, Tc= 133K), mercury (e.g., Hg0.8Tl0.2Ba2Ca2Cu3O8.33, Tc= 138K), or lead-doped (e.g., (Hg0.75Pb0.15Tl0.1)Ba2Ca2Cu3O8+, Tc= 142 K).
Reference
Fahlman, Bradley D. Materials Chemistry. Reprinted 2008. Springer. Pages 38-39.
Monday, April 09, 2012
Mechanism of Water Oxidation to Molecular Oxygen with Osmocene as Photocatalyst: A Theoretical Study
Catalytic water oxidation to molecular oxygen is of fundamental importance to natural and artificial photosynthesis. The direct sunlight-driven splitting of water into O2 and H2 is anticipated as one of the most promising strategies for providing environmentally friendly and renewable energy sources.Photoinduced O2evolution from the [Cp2Os–OH]+ complex in aqueous solution has been studied by the DFT, CASSCF, and CASPT2 methods. The CASPT2//CASSCF calculations predict that the S3 state is initially populated and the subsequent deprotonation of [Cp2Os–OH]+ proceeds very easily along the T1 pathway as a result of the efficient S3 → T1intersystem crossing. It is found that the O–O bond is formed via the acid–base mechanism, which is different from the direct oxo–oxo coupling mechanism suggested in the experimental study. Formation of the O–O bond is the rate-determining step and has an activation energy and activation free energy of 81.3 and 90.4 kcal/mol, respectively. This is consistent with the low quantum yield observed for generating molecular oxygen upon irradiation at 350 nm (aprox.82 kcal/mol). The O2 release from an intermediate complex has to overcome a small barrier on the triplet pathway first and then pass through the triplet–singlet intersection, generating the O2 molecules in either the lowest singlet or triplet state. The formed O2 molecule can be converted into the O2 molecule by the heavy atom effect in the Os complexes, which is probably the reason only the O2molecule was detected experimentally.

Yue Chen (et al.).Mechanism of Water Oxidation to Molecular Oxygen with Osmocene as Photocatalyst: A Theoretical Study. Inorganic Chemistry. American Chemical Society. Apr 1, 2012.http://pubs.acs.org/doi/full/10.1021/ic202097c
From Lithium Bis(trimethylsilyl)amide with Cyanoamine into Triazine Compounds: Synthesis and Structures of Lithium 6-((Trimethylsilyl)amido)-2,4-bis(d
1,3,5-Triazine heterocyclic π-conjugated systems are attractive and versatile for their structures and applications in medicinal chemistry, materials chemistry, and organic synthesis. Accordingly, the 2,4,6-trisubstituted 1,3,5-triazines have attracted much interest. 2,4,6-Triphenyl-1,3,5-triazine derivatives have been largely explored, and pyridyl-substituted 1,3,5-triazines such as 2,4,6-tris(2-pyridyl)-1,3,5-triazines have been extensively referenced. Recently, lanthanide 2,4,6-tris(2-pyridyl)-1,3,5-triazine complexes with visible light luminescence were reported. 2,4,6-Trimercapto-1,3,5-triazine can be used in wastewater treatment to remove heavy metals and also to prepare polynuclear trithiocyanurato-bridged metal complexes bearing bidentate chelating N and S heterocycles. Triprotic 2,4,6-tris(organoamino)-1,3,5-triazenes are promising potential multisite ligands. Moreover, several 1,2,4-triazines, bi(1,2,4-triazines), or transition-metal complexes with disubstituted triazine-based ligands and bi(1,3,5-triazine)ligands were also prepared. However, new and effective syntheses of 1,3,5-triazine heterocyclic rings under mild conditions are still of significance in modern organometallic and organic chemistry; moreover, organometallic derivatives of triazine are scarce and the numbers of accessible heterocyclic skeletons are still limited.
Addition reactions of lithium bis(trimethylsilyl)amide with dimethylcyanamide lead to novel lithium salts of 6-((trimethylsilyl)amido)-2,4-bis(dimethylamino)[1,3,5]triazines [LLi(D)]2 (L = NC(NMe2)NC(NMe2)NC(NSiMe3); D = Me2NCN (1), Et2O (2)) and to the Mn and Co complexes [LL′M] (L′ = N{N(SiMe3)C(NMe2)}2; M = Mn (3), Co (4)). Their formation involves trimethylsilyl shifts, ring formation, and unusual Me2NSiMe3 elimination.


Meisu Zhou (et al.).From Lithium Bis(trimethylsilyl)amide with Cyanoamine into Triazine Compounds: Synthesis and Structures of Lithium 6-((Trimethylsilyl)amido)-2,4-bis(dimethylamino)[1,3,5]triazines and Their Manganese and Cobalt Complexes. Inorganic Chemistry. American Chemical Society. Apr 1, 2012.
Metal–Organic Frameworks with Phosphotungstate Incorporated for Hydrolytic Cleavage of a DNA-Model Phosphodiester

Five phosphotungstate-incorporated metal–organic frameworks
{[Eu4(dpdo)9(H2O)16PW12O40]}(PW12O40)2·(dpdo)3·Cl3 (1); {ZnNa2(μ-
Saturday, April 07, 2012
Iron-Catalyzed Chlorination of Silanes

Iron-Catalyzed Chlorination of Silanes
NEODIMIO ¡no te lo pierdas!

-
Un campo de investigación reciente y muy interesante es el de las máquinas moleculares. Inspirándose en la mecánica biológica, muchos han bu...
-
De entre todos los compuestos químicos que existen, quizá sean los llamados compuestos de coordinación los que mayores dificultades ofrecen ...







