With the recent launch of MIT’s Institute for Medical Engineering and Science, MIT News examines research with the potential to reshape medicine and health care through new scientific knowledge, novel treatments and products, better management of medical data, and improvements in health-care delivery.
Studying infectious diseases has long been primarily the domain of biologists. However, as part of the Ragon Institute, MIT engineers and physical scientists are joining immunologists and physicians in the battle against HIV, which currently infects 34 million people worldwide.
The mission of the Ragon Institute — launched jointly in 2009 by Massachusetts General Hospital (MGH), MIT and Harvard University — is to develop new HIV vaccines through better understanding of how the immune system responds to infection. Bruce Walker, the MGH physician who directs the institute, says it was important to enlist engineers and physical scientists, who have usually been excluded from traditional HIV research, to help in this effort.
“It seemed to me that if we could break down some of those silos, there were probably tools in the toolbox that could be applied to the problem right now that weren’t being applied,” Walker says. “MIT has brought a lot to the table — not only expertise, but also a different way of thinking about approaching problems.”
The Ragon Institute also encourages its researchers to develop new technology and pursue ideas that might not be funded through traditional channels. These include new materials for vaccine delivery and new technology for studying the virus’s interactions with the immune system.
“It has encouraged people, like the engineers here, to start working in areas that they wouldn’t have worked in otherwise,” says Christopher Love, an MIT associate professor of chemical engineering and an associate member of the Ragon Institute. “That kind of momentum can sometimes be hard to establish. The Ragon has been a catalyst for new research innovations and a very effective one at that.”
Single-cell analysis
Love is now helping in the search for a new vaccine using technology he developed to study immune responses of individual cells. His system allows thousands of immune cells to be studied at once: The cells are placed into tiny wells on a plate, and secretions from each cell are imprinted on a glass slide placed over the wells. The slide is then tested for the presence of specific proteins such as cytokines, which provoke inflammation.
Because each cell has its own “address” on the slide, the secretions can be traced back to individual cells. This technology generates a huge amount of data for each cell under study. “You can now make measurements on 10,000 cells and generate 20 to 30 parameters of data on each cell that’s present in that sample. That kind of data density hasn’t really been feasible previously,” says Love, who is a member of MIT's David H. Koch Institute for Integrative Cancer Research.
Love first used the system to study immune-cell responses to food allergens and infectious agents, and began using it to study HIV responses after becoming part of the Ragon Institute in 2009.
In a study published in 2011, Love and his colleagues analyzed the cytokines secreted by T cells from HIV-infected patients, as well as the cells’ ability to kill HIV-infected cells. Previous studies had suggested that high levels of a cytokine called interferon gamma might correlate with cell-killing ability, but the MIT team found that while the percentage of T cells that secrete interferon gamma is similar to the percentage of those that kill infected cells, the populations do not entirely overlap.
Love is now searching for biomarkers that do reveal which T cells are most effective at killing HIV-infected cells. He also hopes to scale up the device so it could be used to rapidly monitor the immune responses of participants in vaccine trials.
New vaccine targets
Arup Chakraborty, director of the Institute for Medical Engineering and Science (IMES) and a professor of chemical engineering, chemistry, physics, and biological engineering at MIT, who uses computational models to study the immune system, had never studied HIV until meeting Walker in 2008. He is now using his computational approaches to seek better HIV vaccine targets.
So far, the virus has proven very difficult to target because it mutates so rapidly. In recent years, scientists have tried targeting amino acids in HIV proteins where mutations appear to weaken the virus. However, this approach has had limited success because compensatory mutations elsewhere in the viral protein can overcome the harmful effects of the vaccine-induced mutation.
To overcome this, Chakraborty’s lab identified groups of amino acids in HIV proteins that evolve independently of those in other groups. In a subset of these groups, computer models predicted the virus to be vulnerable to multiple simultaneous mutations. By targeting amino acids in such groups, vaccine designers may be able to cut off the virus’s escape route.
In 2011, Chakraborty and Walker showed that a particularly vulnerable group exists in a subunit of the Gag protein, which forms the envelope that surrounds the virus’s genetic material. They also found that T cells in patients who can fight off HIV on their own disproportionately target the amino acids identified in the study. HIV strains with multiple mutations in these amino acids are rare, offering further evidence that these could make good vaccine targets.
Special delivery
Darrell Irvine, an MIT professor of materials science and engineering and member of the Koch Institute, is working on alternative ways to deliver vaccines. Most vaccines used to protect against diseases such as chicken pox and influenza are made from deactivated forms of the virus. That approach is thought too risky for HIV, so many researchers are instead pursuing vaccines made from protein or sugar molecules that the virus produces, known as antigens. Another possible approach is injecting DNA that codes for viral proteins.
However, injecting those molecules on their own doesn’t always produce a strong-enough immune response in the vaccine recipient, so Irvine and his lab are seeking ways to elicit stronger responses, using two strategies: delivering antigen along with another type of molecule, known as an adjuvant, that helps to provoke the immune system, and delivering the antigen directly to the target cells, using nanoparticles or polymer films.
Recently, Irvine and his colleagues developed a new polymer film that can deliver DNA vaccines under the skin. DNA vaccines were first tested about 20 years ago, and found to elicit strong immune responses in rodents. However, DNA vaccines have thus far failed to provoke any protective response in human clinical trials.
With the new polymer film developed by Irvine and his colleagues, DNA vaccines are embedded in layers of polymer films that gradually degrade, releasing the vaccine over days or weeks. The film also includes an adjuvant consisting of a strand of RNA similar to viral RNA. This molecule provokes inflammation in the target tissue, which helps to recruit immune cells to the area, so they can encounter the antigen encoded by the DNA.
The vaccine-delivering film showed success in tests of mice, and the researchers now hope to test it in nonhuman primates.
Much of this work would probably never have happened without both funding from the Ragon Institute and the interdisciplinary collaborations that have arisen because of the institute.
“It’s been absolutely fantastic for me and many of the MIT faculty that have been involved,” Irvine says. “There are really two paths being followed at all times: a very focused mission to try and get an HIV vaccine developed, but also an interest in making sure that we don’t miss new opportunities in the basic science that might bring totally new vaccine concepts forward.”
Source: http://web.mit.edu

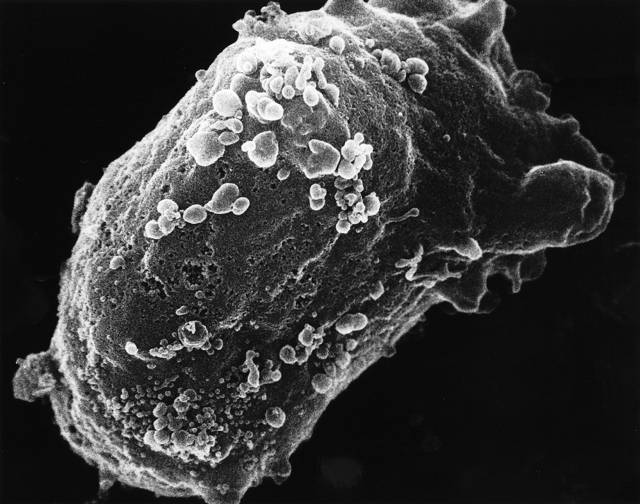
Scanning electron microscope image of a lymphocyte with HIV cluster. (Image: National Cancer Institute)
Studying infectious diseases has long been primarily the domain of biologists. However, as part of the Ragon Institute, MIT engineers and physical scientists are joining immunologists and physicians in the battle against HIV, which currently infects 34 million people worldwide.
The mission of the Ragon Institute — launched jointly in 2009 by Massachusetts General Hospital (MGH), MIT and Harvard University — is to develop new HIV vaccines through better understanding of how the immune system responds to infection. Bruce Walker, the MGH physician who directs the institute, says it was important to enlist engineers and physical scientists, who have usually been excluded from traditional HIV research, to help in this effort.
“It seemed to me that if we could break down some of those silos, there were probably tools in the toolbox that could be applied to the problem right now that weren’t being applied,” Walker says. “MIT has brought a lot to the table — not only expertise, but also a different way of thinking about approaching problems.”
The Ragon Institute also encourages its researchers to develop new technology and pursue ideas that might not be funded through traditional channels. These include new materials for vaccine delivery and new technology for studying the virus’s interactions with the immune system.
“It has encouraged people, like the engineers here, to start working in areas that they wouldn’t have worked in otherwise,” says Christopher Love, an MIT associate professor of chemical engineering and an associate member of the Ragon Institute. “That kind of momentum can sometimes be hard to establish. The Ragon has been a catalyst for new research innovations and a very effective one at that.”
Single-cell analysis
Love is now helping in the search for a new vaccine using technology he developed to study immune responses of individual cells. His system allows thousands of immune cells to be studied at once: The cells are placed into tiny wells on a plate, and secretions from each cell are imprinted on a glass slide placed over the wells. The slide is then tested for the presence of specific proteins such as cytokines, which provoke inflammation.
Because each cell has its own “address” on the slide, the secretions can be traced back to individual cells. This technology generates a huge amount of data for each cell under study. “You can now make measurements on 10,000 cells and generate 20 to 30 parameters of data on each cell that’s present in that sample. That kind of data density hasn’t really been feasible previously,” says Love, who is a member of MIT's David H. Koch Institute for Integrative Cancer Research.
Love first used the system to study immune-cell responses to food allergens and infectious agents, and began using it to study HIV responses after becoming part of the Ragon Institute in 2009.
In a study published in 2011, Love and his colleagues analyzed the cytokines secreted by T cells from HIV-infected patients, as well as the cells’ ability to kill HIV-infected cells. Previous studies had suggested that high levels of a cytokine called interferon gamma might correlate with cell-killing ability, but the MIT team found that while the percentage of T cells that secrete interferon gamma is similar to the percentage of those that kill infected cells, the populations do not entirely overlap.
Love is now searching for biomarkers that do reveal which T cells are most effective at killing HIV-infected cells. He also hopes to scale up the device so it could be used to rapidly monitor the immune responses of participants in vaccine trials.
New vaccine targets
Arup Chakraborty, director of the Institute for Medical Engineering and Science (IMES) and a professor of chemical engineering, chemistry, physics, and biological engineering at MIT, who uses computational models to study the immune system, had never studied HIV until meeting Walker in 2008. He is now using his computational approaches to seek better HIV vaccine targets.
So far, the virus has proven very difficult to target because it mutates so rapidly. In recent years, scientists have tried targeting amino acids in HIV proteins where mutations appear to weaken the virus. However, this approach has had limited success because compensatory mutations elsewhere in the viral protein can overcome the harmful effects of the vaccine-induced mutation.
To overcome this, Chakraborty’s lab identified groups of amino acids in HIV proteins that evolve independently of those in other groups. In a subset of these groups, computer models predicted the virus to be vulnerable to multiple simultaneous mutations. By targeting amino acids in such groups, vaccine designers may be able to cut off the virus’s escape route.
In 2011, Chakraborty and Walker showed that a particularly vulnerable group exists in a subunit of the Gag protein, which forms the envelope that surrounds the virus’s genetic material. They also found that T cells in patients who can fight off HIV on their own disproportionately target the amino acids identified in the study. HIV strains with multiple mutations in these amino acids are rare, offering further evidence that these could make good vaccine targets.
Special delivery
Darrell Irvine, an MIT professor of materials science and engineering and member of the Koch Institute, is working on alternative ways to deliver vaccines. Most vaccines used to protect against diseases such as chicken pox and influenza are made from deactivated forms of the virus. That approach is thought too risky for HIV, so many researchers are instead pursuing vaccines made from protein or sugar molecules that the virus produces, known as antigens. Another possible approach is injecting DNA that codes for viral proteins.
However, injecting those molecules on their own doesn’t always produce a strong-enough immune response in the vaccine recipient, so Irvine and his lab are seeking ways to elicit stronger responses, using two strategies: delivering antigen along with another type of molecule, known as an adjuvant, that helps to provoke the immune system, and delivering the antigen directly to the target cells, using nanoparticles or polymer films.
Recently, Irvine and his colleagues developed a new polymer film that can deliver DNA vaccines under the skin. DNA vaccines were first tested about 20 years ago, and found to elicit strong immune responses in rodents. However, DNA vaccines have thus far failed to provoke any protective response in human clinical trials.
With the new polymer film developed by Irvine and his colleagues, DNA vaccines are embedded in layers of polymer films that gradually degrade, releasing the vaccine over days or weeks. The film also includes an adjuvant consisting of a strand of RNA similar to viral RNA. This molecule provokes inflammation in the target tissue, which helps to recruit immune cells to the area, so they can encounter the antigen encoded by the DNA.
The vaccine-delivering film showed success in tests of mice, and the researchers now hope to test it in nonhuman primates.
Much of this work would probably never have happened without both funding from the Ragon Institute and the interdisciplinary collaborations that have arisen because of the institute.
“It’s been absolutely fantastic for me and many of the MIT faculty that have been involved,” Irvine says. “There are really two paths being followed at all times: a very focused mission to try and get an HIV vaccine developed, but also an interest in making sure that we don’t miss new opportunities in the basic science that might bring totally new vaccine concepts forward.”
Source: http://web.mit.edu



No comments:
Post a Comment