| (Nanowerk News) In the search for efficient, durable and commercially viable fuel cells, scientists at the University of Ulster's Nanotechnology Institute and collaborators from Peking University and University of Oxford have discovered a new catalyst-support combination that could make fuel cells more efficient and more resistant to carbon monoxide, CO, poisoning. The research – described in The Journal of Physical Chemistry C ("Rapid Microwave Synthesis of CO Tolerant Reduced Graphene Oxide-Supported Platinum Electrocatalysts for Oxidation of Methanol") – may expedite the realisation of fuel-cell vehicles. | |
| Fuel cells that convert chemical energy directly into electrical energy by electrochemically decomposing a fuel, such as hydrogen or methanol are considered a promising new way of powering cars and portable devices. One major hurdle to the commercial use of fuel cells is the CO poisoning of the active platinum catalyst sites, which renders them ineffective and prevents fuel oxidation. The CO poisoning problem is especially severe in direct methanol fuel cells (DMFCs) because CO is always present in critical amounts as an intermediate in methanol oxidation reaction. So far the major approach for reducing the poisoning is to alloy platinum with other expensive metals such as Ru, Pd or Au. | |
| However, now Ulster scientists have found a cheaper solution that could help bring fuel cell devices a step closer to the market. To create a catalyst system that can tolerate more carbon monoxide, they deposited platinum nanocrystals on a support material of graphene oxide and reduced it slightly to increase its electrical conductivity. They used a simple scalable, fast and eco-friendly microwave approach that has the advantage of reducing graphene oxide (RGO) and forming platinum nanoparticles simultaneously. To test the activity of the Pt/RGO the team looked at the oxidation of methanol - a reaction that takes place at the anode of a methanol fuel cell. Their research shows that the new material displays an unprecedented CO poisoning tolerance, a much better long term stability and a higher electrocatalytic activity than those exhibited by commercially available carbon-supported Pt (Pt/C) electrocatalysts. | |
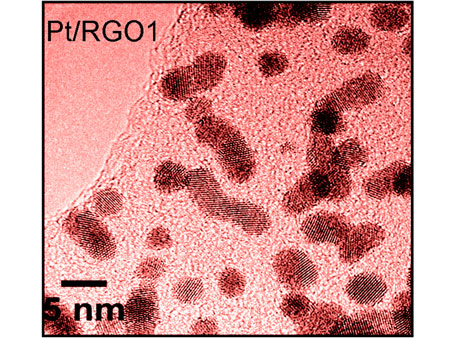 | |
| Platinum nanocatalysts supported on lightly reduced graphene oxide could make fuel cells more stable and resistant to carbon monoxide poisoning. | |
| Structural and electronic properties of the electrocatalysts were determined using high resolution X ray photoelectron spectroscopy at the National Centre for Electron Spectroscopy and Surface Analysis (NCESS) at Daresbury, combined with transmission electron microscopy analysis at the EPSRC funded TEM facility at Oxford university. | |
| "Our studies of the structure and activity of this catalyst -- and comparisons with commercial Pt/C catalysts currently in use -- illustrate that the lightly reduced graphene oxide support 'protects' the fine platinum nanocrystals from CO poisoning, enabling them to exhibit long term operation stability" explained Ulster Professor of Advanced Materials, Pagona Papakonstantinou, who leads the research team at Ulster University. | |
| The abundance of residual oxygen groups on lightly reduced graphene oxide (RGO) plays a major role on the removal of carbonaceous species. When an electrochemical potential is applied to the electrode, water molecules on the RGO support dissociate to form -(OH) groups, which readily oxidize the CO adsorbed groups on the adjacent Pt sites. 'This is one probable mechanism, we believe is operating to provide CO free Pt sites' | |
| It is important to emphasize that the team has come up with a new electrocatalyst design that can be considered as a promising alternative for improving durability of fuel cells and eliminate the use of costly bimetallic or ternary metal systems. | |
| The research was support by a Leverhulme Trust visiting Fellowship (Dr A. Ganguly) and a VCRS PhD studentship (S. Sharma). |
| Source: University of Ulster |



No comments:
Post a Comment